Subtotal: $67.00
GHB
GBL
GBL
GHB
LSD Acid
LSD Acid
Shrooms Microdose Capsules
Dmt for sale
Shrooms Microdose Capsules
Dmt for sale
Magic Mushrooms
LSD Acid
Magic Mushrooms
ghb
what is ghb?
gamma-Hydroxybutyric acid or γ-Hydroxybutyric acid (GHB), also known as 4-hydroxybutanoic acid, is a naturally occurring neurotransmitter and a psychoactive drug. It is a precursor to GABA, glutamate, and glycine in certain brain areas. It acts on the GHB receptor and is a weak agonist at the GABAB receptor. GHB has been used in the medical setting as a general anesthetic and as a treatment for cataplexy, narcolepsy, and alcoholism. It is also used illegally as an intoxicant, as an athletic-performance enhancer, as a date-rape drug, and as a recreational drug.
It is commonly used in the form of a salt, such as sodium γ-hydroxybutyrate (NaGHB, sodium oxybate, or Xyrem) or potassium γ-hydroxybutyrate (KGHB, potassium oxybate). GHB is also produced as a result of fermentation, and is found in small quantities in some beers and wines, beef and small citrus fruits.
Medical use of buy ghb
gamma-Hydroxybutyric acid or γ-Hydroxybutyric acid is used for medical purposes in the treatment of narcolepsy and, more rarely, alcoholism, although there remains uncertainty about its efficacy relative to other pharmacotherapies for alcohol dependence, buy ghb. It is sometimes used off-label for the treatment of fibromyalgia. gamma-Hydroxybutyric acid or γ-Hydroxybutyric acid is the active ingredient of the prescription medication sodium oxybate (Xyrem). Sodium oxybate is approved by U.S. Food and Drug Administration for the treatment of cataplexy associated with narcolepsy and excessive daytime sleepiness (EDS) associated with narcolepsy.
gamma-Hydroxybutyric acid or γ-Hydroxybutyric acid has been shown to reliably increase slow-wave sleep and decrease the tendency for REM sleep in modified multiple sleep latency tests.
The FDA approved labeling for sodium oxybate suggests no evidence gamma-Hydroxybutyric acid or γ-Hydroxybutyric acid has teratogenic, carcinogenic or hepatotoxic properties. Its favorable safety profile relative to ethanol may explain why GHB continues to be investigated] as a candidate for alcohol substitution.
ghb effects
Adverse effects{ghb side effects}
In humans, this product has been shown to reduce the elimination rate of alcohol. This may explain the respiratory arrest that has been reported after ingestion of both drugs. A review of the details of 194 deaths attributed to or related to gamma-Hydroxybutyric acid or γ-Hydroxybutyric acid over a ten-year period found that most were from respiratory depression caused by interaction with alcohol or other drugs. Combination with alcohol
Deaths
One publication has investigated 226 deaths attributed to GHB. Of 226 deaths included, 213 had a cardiorespiratory arrest and 13 had fatal accidents. Seventy-one deaths (34%) had no co-intoxicants. Postmortem blood GHB was 18–4400 mg/L (median=347) in deaths negative for co-intoxicants.
One report has suggested that sodium oxybate overdose might be fatal, based on deaths of three patients who had been prescribed the drug. However, for two of the three cases, post-mortem GHB concentrations were 141 and 110 mg/L, which is within the expected range of concentrations for gamma-Hydroxybutyric acid or γ-Hydroxybutyric acid after death, and the third case was a patient with a history of intentional drug overdose. The toxicity of gamma-Hydroxybutyric acid or γ-Hydroxybutyric acid has been an issue in criminal trials, as in the death of Felicia Tang, where the defense argued that death was due to GHB, not murder.
gamma-Hydroxybutyric acid or γ-Hydroxybutyric acid is produced in the body in very small amounts, and blood levels may climb after death to levels in the range of 30–50 mg/L. Levels higher than this are found in GHB deaths. Levels lower than this may be due to GHB or to postmortem endogenous elevations.
Neurotoxicity
In multiple studies, gamma-Hydroxybutyric acid or γ-Hydroxybutyric acid has been found to impair spatial memory, working memory, learning and memory in rats with chronic administration. These effects are associated with decreased NMDA receptor expression in the cerebral cortex and possibly other areas as well. In addition, the neurotoxicity appears to be caused by oxidative stress.
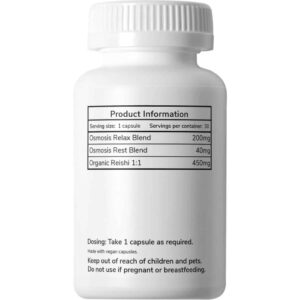
 Focus Mushroom Capsules 100mg
Focus Mushroom Capsules 100mg